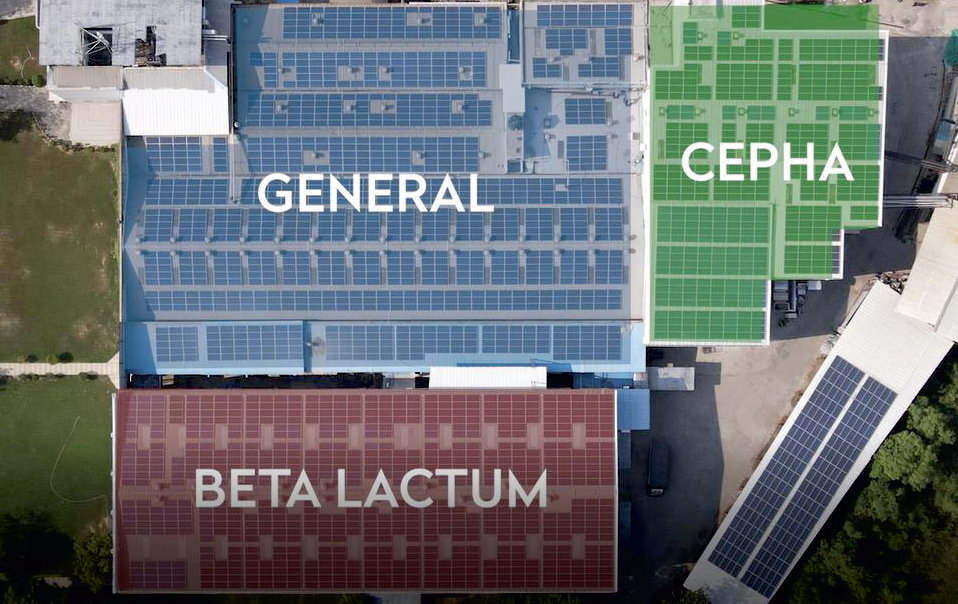
GENERAL
A versatile production facility handling a wide range of formulations across therapeutic categories, built to support both domestic and international markets.
- WHO-GMP compliant for domestic and export markets
- Oral Liquid Line: Flexible packaging for PET & Glass bottles
- Injectables (SVP): Fully automated lines with funnel system & visual inspector
- Flexible Packaging Options: For tablets, capsules, ointments & creams

Capsules
1120 Million
(Annual Production Capacity)

Tablets
1845 Million
(Annual Production Capacity)

Ointment & Cream
46 Million
(Annual Production Capacity)
Injections (amp & vials)
103 Million
(Annual Production Capacity)

Oral Liquids
46 Million
(Annual Production Capacity)

27 Million
(Annual Production Capacity)

Powder Sachets
27 Million
(Annual Production Capacity)
BETA LACTUM
Beta Lactam Antibiotics production plant features high safety equipment & a comprehensive record of global GMP inspections.
- Automated Manufacturing: Handles tablets, capsules, dry syrups, powder for injections, and global packaging configurations
- Material Handling Systems: Reduces contamination and handling loss for injections

Capsules
900 Million
(Annual Production Capacity)

Tablets
900 Million
(Annual Production Capacity)

Dry Syrup
46 Million
(Annual Production Capacity)

Powder for Injection
45 Million
(Annual Production Capacity)
Cephalosporin Block
The cephalosporin block is an extensively controlled sterile block with highest quality compliant standards.
- Dry Powder for Injection: Fully automated packaging with Auto Cartonator
- Material Handling Systems: Minimizes contamination and handling loss
- Advanced Equipment: Includes automatic coating for tablets, barcoding for injections, BQS machine for blister packing, and humidity control for sensitive products

Capsules
518 Million
(Annual Production Capacity)

Tablets
660 Million
(Annual Production Capacity)

Dry Syrup
51 Million
(Annual Production Capacity)

Powder for Injection
45 Million
(Annual Production Capacity)
Quality You Can Trust, Systems You Can Rely On
Our WHO-GMP certified facility in Vadodara is a fully integrated, self-reliant unit equipped for sterile and other/oral dosage manufacturing. With in-house F&D, ADL, QC, QA (including IP-QA), Regulatory, and Engineering teams, we ensure precision at every step. Dedicated HVAC systems, GLP-certified labs, and robust ERP systems power seamless operations — from sourcing to batch release — serving both domestic and international markets with unwavering quality and control.
Globally Certified for
Quality and
Compliance
























Sustainable Manufacturing in Action
At Bharat Parenterals Limited, sustainability is not an afterthought — it’s a commitment built into how we manufacture, operate, and grow. We actively reduce our environmental footprint through clean energy and resource efficiency.

1050 KVA Solar Panel Installation
across 92,536 sq. ft.

ZERO Discharge Wastewater Facility
saving 20,000 liters daily

500+ Trees Planted
and maintained through annual drives

On-Site Organic Farming
for employees and biodiversity